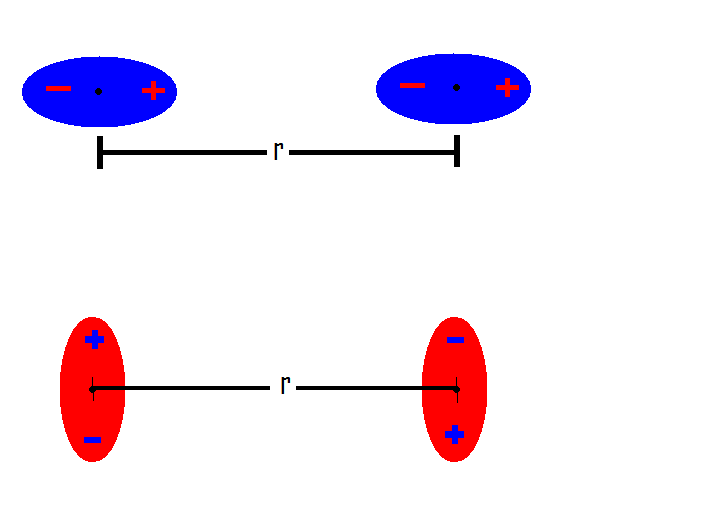
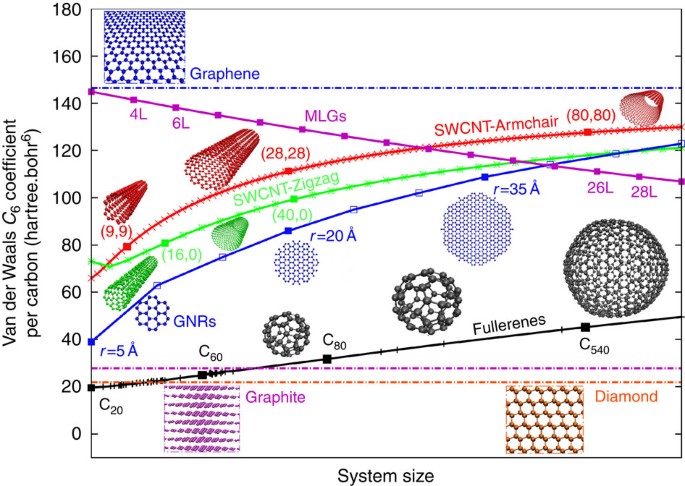
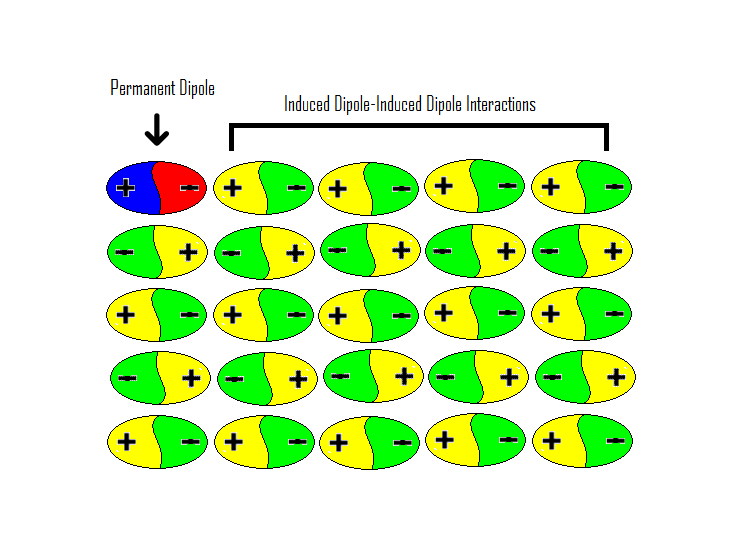
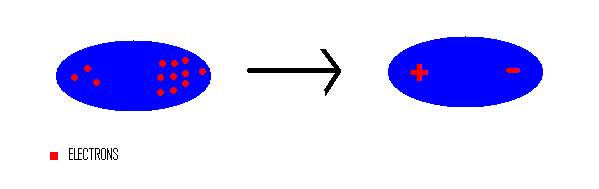
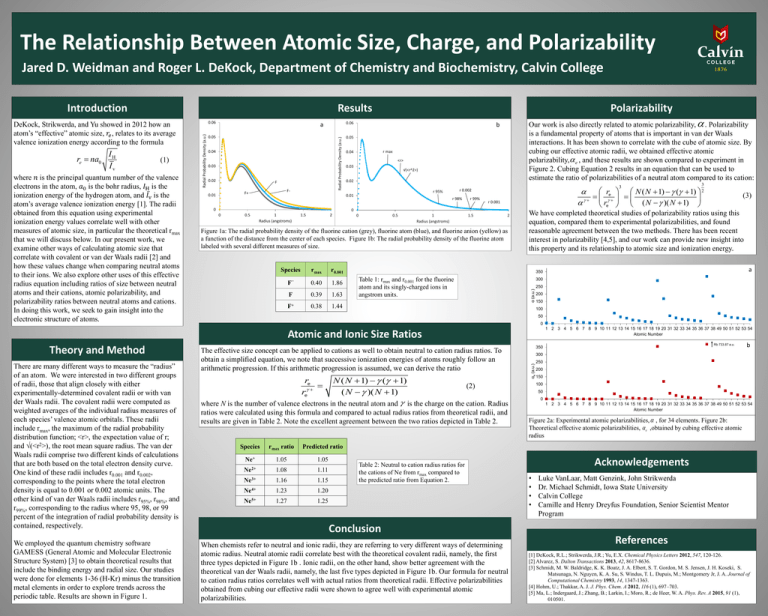
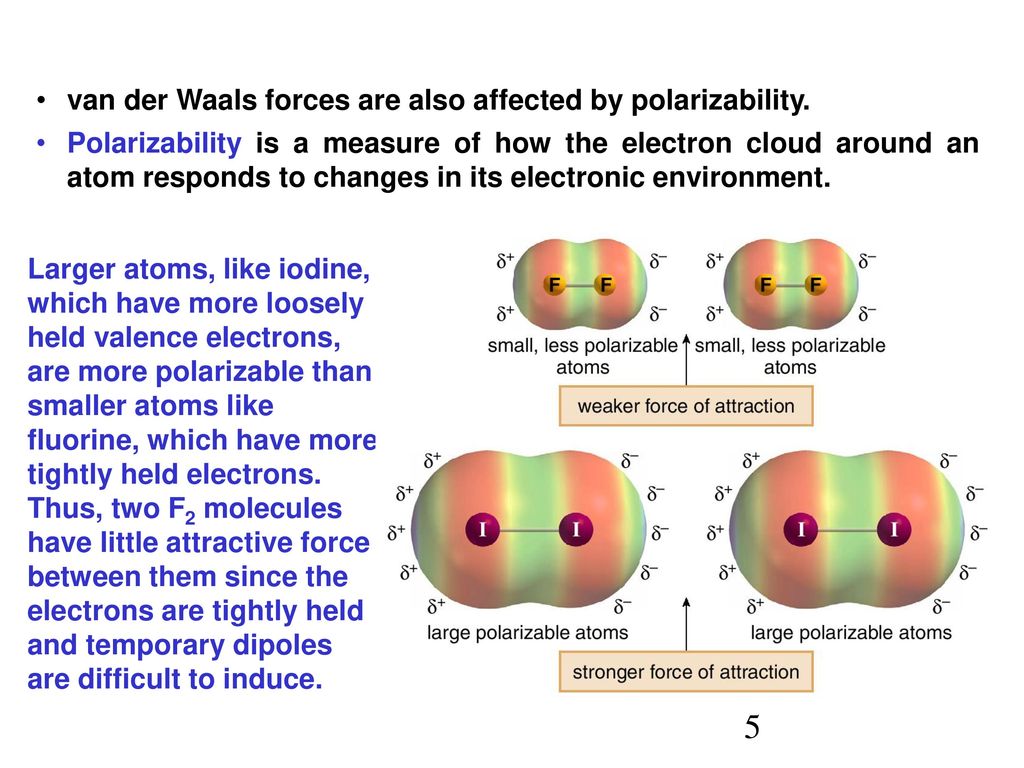
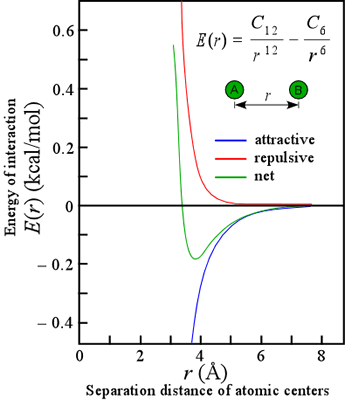
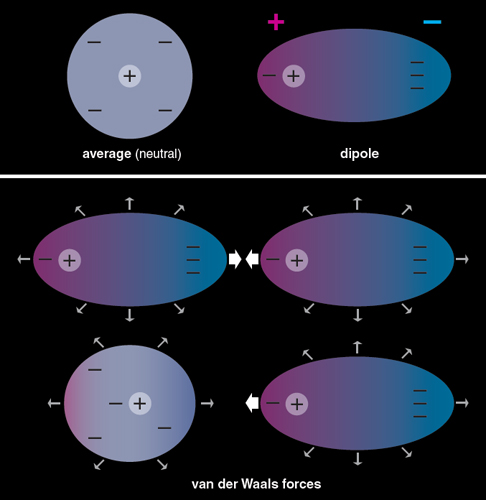
Communication: Non-additivity of van der Waals interactions between nanostructures: The Journal of Chemical Physics: Vol 141, No 14
![PDF] Theoretical investigation of the potential energy, dipole moment and polarizability surfaces of the CH4 - N2 and C2H4 - C2H4 van der Waals complexes | Semantic Scholar PDF] Theoretical investigation of the potential energy, dipole moment and polarizability surfaces of the CH4 - N2 and C2H4 - C2H4 van der Waals complexes | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/606eadef63b76ddc96930bb6b88cf6a27beb68dd/60-Figure3.1-1.png)
PDF] Theoretical investigation of the potential energy, dipole moment and polarizability surfaces of the CH4 - N2 and C2H4 - C2H4 van der Waals complexes | Semantic Scholar
Effects of van der Waals interactions on the polarizability of atoms, oscillators, and dipolar rotors at long range: The Journal of Chemical Physics: Vol 75, No 6
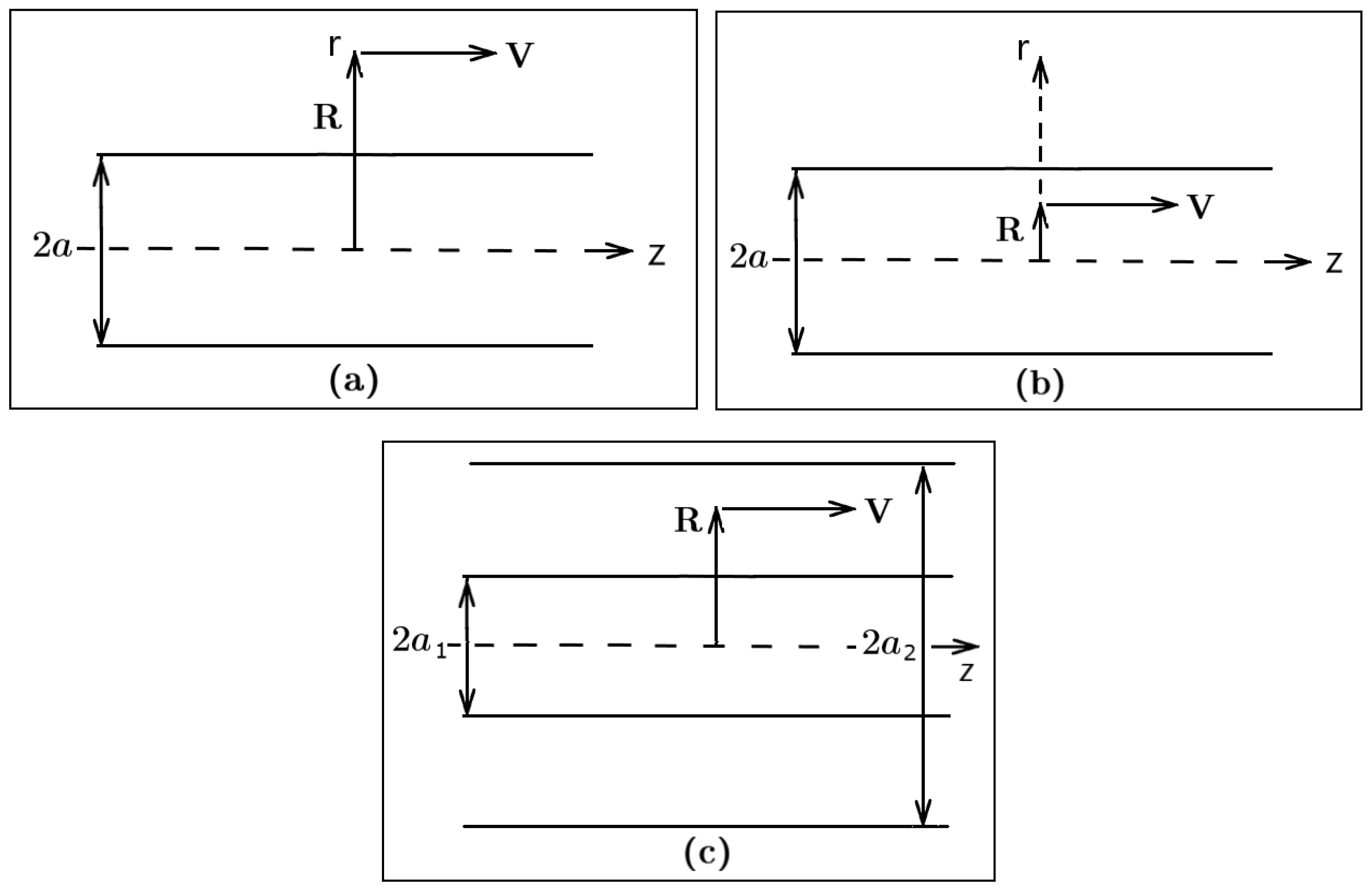
Universe | Free Full-Text | Van der Waals Interactions of Moving Particles with Surfaces of Cylindrical Geometry | HTML

van der Waals coefficients C6 (in Ry atomic units) calculated from E... | Download Scientific Diagram
![PDF] Theoretical investigation of the potential energy, dipole moment and polarizability surfaces of the CH4 - N2 and C2H4 - C2H4 van der Waals complexes | Semantic Scholar PDF] Theoretical investigation of the potential energy, dipole moment and polarizability surfaces of the CH4 - N2 and C2H4 - C2H4 van der Waals complexes | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/606eadef63b76ddc96930bb6b88cf6a27beb68dd/21-Figure1.1-1.png)
PDF] Theoretical investigation of the potential energy, dipole moment and polarizability surfaces of the CH4 - N2 and C2H4 - C2H4 van der Waals complexes | Semantic Scholar
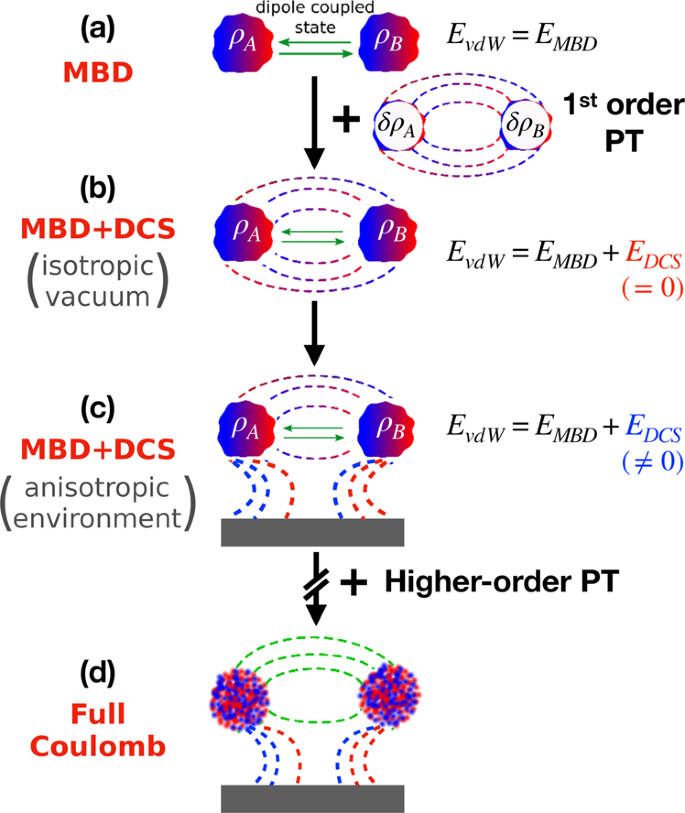
Coulomb interactions between dipolar quantum fluctuations in van der Waals bound molecules and materials | Nature Communications

Frequency-dependent polarizabilities of diatomic molecules: Density functional theory and ab initio methods compared with quantum-defect Green function technique - ScienceDirect
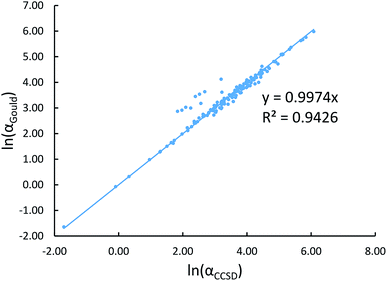
New scaling relations to compute atom-in-material polarizabilities and dispersion coefficients: part 1. Theory and accuracy - RSC Advances (RSC Publishing) DOI:10.1039/C9RA03003D

Insight into induced charges at metal surfaces and biointerfaces using a polarizable Lennard–Jones potential | Nature Communications